Newsroom
iHealth to Offer Savings Up to 63% on Best Sellers During Amazon October Prime Big Deal Days
iHealth is offering savings up to as high as 63% off for the Amazon October Prime Big Deals Days on October 10 & 11. iHealth’s Big Deal products include its #1 best-selling no-touch thermometer and highly-rated at-home Covid antigen rapid test. As a featured brand partner of Amazon Buy with Prime in the Wellness category, iHealth also offers Prime shipping on its DTC (direct to consumer site), on a curated collection of products.
Read MoreiHealth Labs Continues Rapid Growth of Unified Care Program with Sacramento Office Opening
iHealth Labs has opened a new office in Sacramento at 8950 Cal Center Drive to accommodate its rapidly growing Unified Care program. With its contributions as a pivotal COVID-19 home test provider and a decade-long dedication to reimagining chronic disease care, the company is poised to amplify its impact for population health with the expansion of the Unified Care program in Northern California, especially the Greater Sacramento Area.
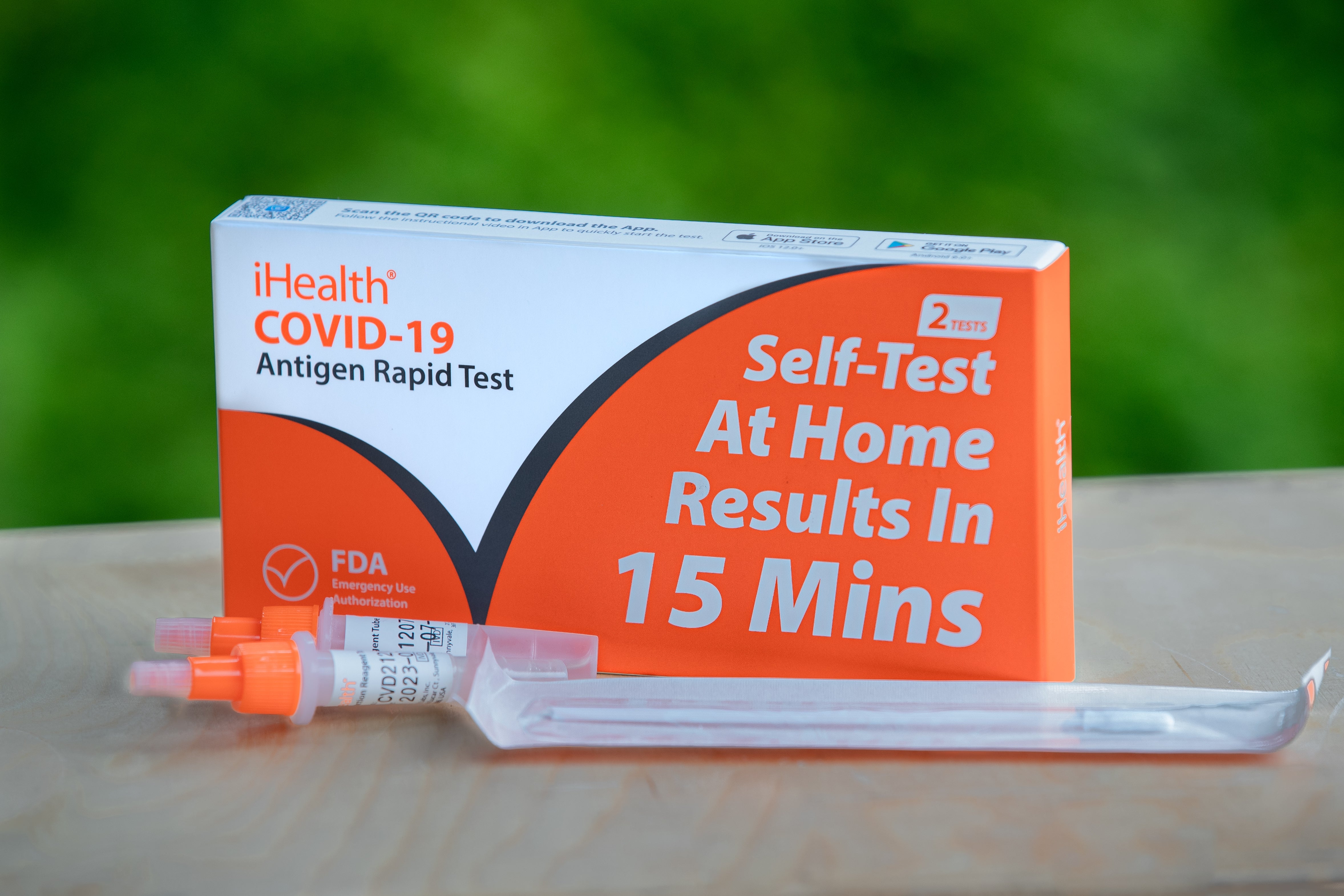
Read MoreiHealth Manufactures Over 70 Million iHealth COVID-19 Test Kits in Irwindale, CA
iHealth's manufacturing facility for COVID-19 tests in Irwindale, CA has manufactured over 70 million test kits to-date. The iHealth team has made a substantial investment to establish this US-based production facility for COVID-19 at-home tests. The facility, which opened in August 2022, spans 57,800 square feet and boasts 32 production lines. iHealth has created hundreds of local jobs and has production capacity of up to one million tests per day.
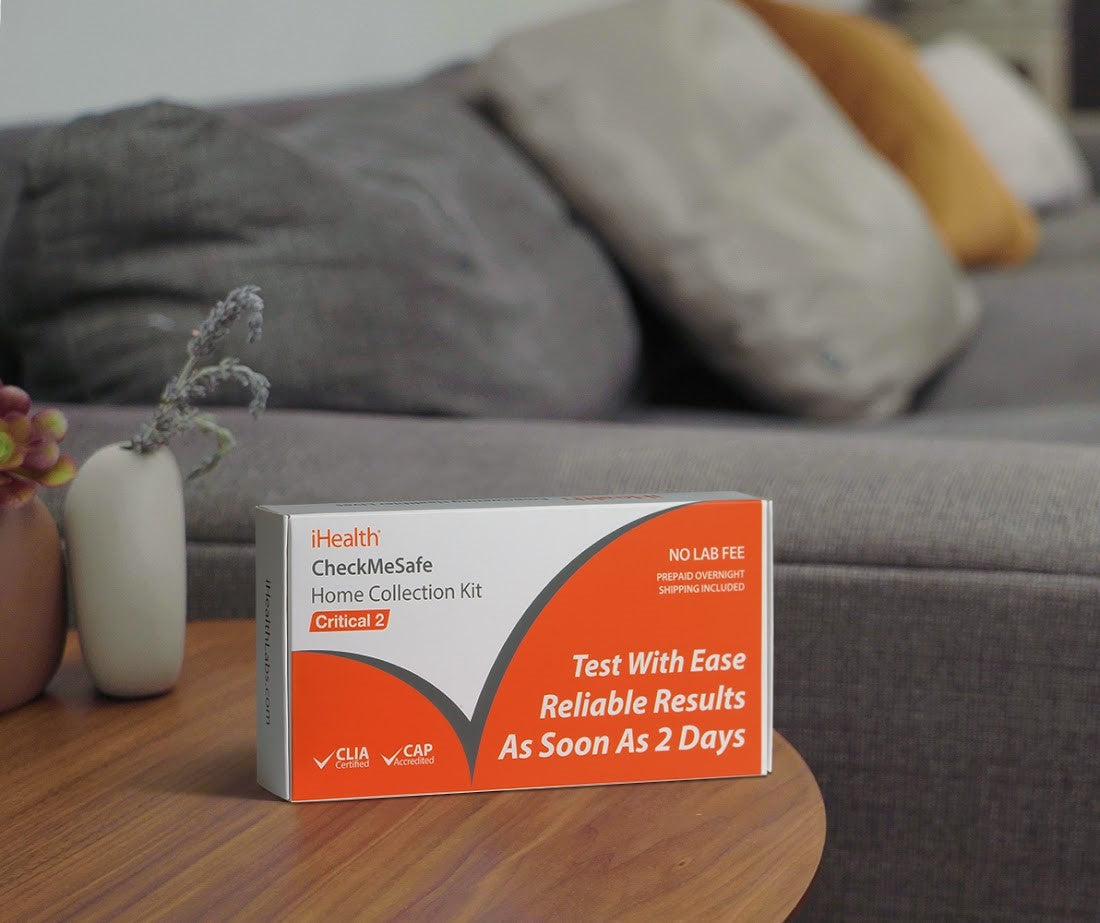
Read MoreiHealth Launches Home Collection Kit for STD Testing
iHealth launched the CheckMeSafe Home Collection kit, its first in the STD OTC test category, in response to the growing STD crisis in the US. The CDC has called for more groups from local, healthcare industry and public health sectors to contribute to STI prevention and innovation efforts. The FDA-approved CheckMeSafe Home Collection kit delivers reliable results of up to 99.9% sensitivity for HIV and 99.5% for syphilis.
Read MoreiHealth Donates 1-Million At-Home Tests to the State of California
iHealth Labs, Inc., one of the major suppliers of at-home COVID tests in the U.S. market, is donating 1 million at-home COVID tests to the State of California. This donation will provide California's residents with much-needed support as another surge in coronavirus infections in the state has led to high demand for early detection.
Read MoreiHealth's Super Bowl Debut Highlights Its Commitment to Bring More Tests to More People ASAP
iHealth Labs, Inc. made its Super Bowl debut with in-stadium advertising highlighting its commitment to bring at-home COVID-19 tests to more people as quickly as possible. iHealth's Super Bowl advertising featured LED ribbon takeovers and branded openings on the stadium's video board that ran during the breaks at the end of the first and third quarters on Sunday, Feb. 13 at SoFi Stadium.
Read MoreiHealth Statement on the COVID-19 Omicron Variant and Its At-Home Test Kit's Effectiveness
iHealth has been continuously monitoring the COVID-19 variants so the iHealth COVID-19 Antigen Rapid Test is ready to address any significant changes in the epidemic. We are confident that our rapid antigen test continues to detect COVID-19 infection, including infection with the Omicron variant.
Read More